library(ComplexHeatmap)
library(EnrichedHeatmap)
library(SummarizedExperiment)
library(gUtils)
library(rtracklayer)
library(data.table)
library(parallel)
library(R.utils)
library(circlize)
library(dplyr)
library(tibble)Exercise 4
Overlapping all the datasets
Learning Objectives
By the end of this exercise, you will be able to: - Load and explore genomic data using Bioconductor classes such as GRangesList and RangedSummarizedExperiment. - Subset and filter genomic features based on annotation or genomic coordinates. - Perform basic overlap and distance-based queries between genomic intervals. - Integrate multi-assay data (e.g., chromatin marks and gene expression) using shared genomic coordinates.
Load Libraries
SE objects
atac <- readRDS("data/atac_se.rds")
rna <- readRDS("data/rna_se.rds")
h3k4me3 <- readRDS("data/h3k4me3_se.rds")
h3k4me1 <- readRDS("data/h3k4me1_se.rds")
h3k27me3 <- readRDS("data/h3k27me3_se.rds")
h3k27ac <- readRDS("data/h3k27ac_se.rds")Overlap matrix
To make the overlapMatrix (you could also say overlapRanges), we need to identify a target.
Function to merge Genomic Ranges
# Merge metadata from two GRanges objects based on overlaps
metaGR <- function(gr1, gr2, minOverlap = 1) {
# Initialize metadata with gr1's metadata
mcols_out <- mcols(gr1)
# Find overlaps (keep all from gr1)
hits <- findOverlaps(gr1, gr2, minoverlap = minOverlap)
idx1 <- subjectHits(hits)
idx2 <- queryHits(hits)
# Prepare new metadata to be added
new_mcols <- DataFrame(matrix(NA, nrow = length(gr1), ncol = ncol(mcols(gr2))))
colnames(new_mcols) <- colnames(mcols(gr2))
# Fill metadata only for overlaps
new_mcols[idx2, ] <- mcols(gr2)[idx1, , drop = FALSE]
# Combine original and new metadata
mcols(gr1) <- cbind(mcols_out, new_mcols)
return(gr1)
}ATAC-Seq
# Extract rowRanges and filter peaks with strong signal
rd_atac <- rowRanges(atac)
rd_atac <- rd_atac[abs(rd_atac$logFC) >= 0.5 & rd_atac$qvalue <= 0.1]
colnames(elementMetadata(rd_atac)) <- paste("ATAC", colnames(elementMetadata(rd_atac)), sep = "_")RNA
# Filter differentially expressed genes
rd_rna <- rowRanges(rna)
rd_rna <- rd_rna[abs(rd_rna$logFC) >= 0.5 & rd_rna$qvalue <= 0.1]
colnames(elementMetadata(rd_rna)) <- paste("RNA", colnames(elementMetadata(rd_rna)), sep = "_")
overlap <- metaGR(gr1 = rd_atac, gr2 = rd_rna, minOverlap = 10)ChIP-Seq
H3K4me3
rd_h3k4me3 <- rowRanges(h3k4me3)
rd_h3k4me3 <- rd_h3k4me3[abs(rd_h3k4me3$logFC) >= 0.5 & rd_h3k4me3$qvalue <= 0.1]
colnames(elementMetadata(rd_h3k4me3)) <- paste("H3K4me3", colnames(elementMetadata(rd_h3k4me3)), sep = "_")
overlap <- metaGR(gr1 = overlap, gr2 = rd_h3k4me3, minOverlap = 10)H3K4me1
rd_h3k4me1 <- rowRanges(h3k4me1)
rd_h3k4me1 <- rd_h3k4me1[abs(rd_h3k4me1$logFC) >= 0.5 & rd_h3k4me1$qvalue <= 0.1]
colnames(elementMetadata(rd_h3k4me1)) <- paste("H3K4me1", colnames(elementMetadata(rd_h3k4me1)), sep = "_")
overlap <- metaGR(gr1 = overlap, gr2 = rd_h3k4me1, minOverlap = 10)H3K27me3
rd_h3k27me3 <- rowRanges(h3k27me3)
rd_h3k27me3 <- rd_h3k27me3[abs(rd_h3k27me3$logFC) >= 0.5 & rd_h3k27me3$qvalue <= 0.1]
colnames(elementMetadata(rd_h3k27me3)) <- paste("H3K27me3", colnames(elementMetadata(rd_h3k27me3)), sep = "_")
overlap <- metaGR(gr1 = overlap, gr2 = rd_h3k27me3, minOverlap = 10)H3K27ac
rd_h3k27ac <- rowRanges(h3k27ac)
rd_h3k27ac <- rd_h3k27ac[abs(rd_h3k27ac$logFC) >= 0.5 & rd_h3k27ac$qvalue <= 0.1]
colnames(elementMetadata(rd_h3k27ac)) <- paste("H3K27ac", colnames(elementMetadata(rd_h3k27ac)), sep = "_")
overlap <- metaGR(gr1 = overlap, gr2 = rd_h3k27ac, minOverlap = 10)Save data
# Add a name field and save the final integrated object
overlap$name <- paste("peak", 1:length(overlap), sep = "_")
names(overlap) <- overlap$name
dir.create("output", showWarnings = FALSE)
saveRDS(object = overlap, file = "output/overlap_data.rds")Snapshot of overlaMatrix
overlap[1:3]GRanges object with 3 ranges and 109 metadata columns:
seqnames ranges strand | ATAC_annotation ATAC_geneChr
<Rle> <IRanges> <Rle> | <character> <integer>
peak_1 chr1 3670547-3672665 * | Promoter (<=1kb) 1
peak_2 chr1 4332510-4332710 * | Intron (ENSMUST00000.. 1
peak_3 chr1 4491755-4492573 * | Promoter (1-2kb) 1
ATAC_geneStart ATAC_geneEnd ATAC_geneLength ATAC_geneStrand
<integer> <integer> <integer> <integer>
peak_1 3214482 3671498 457017 2
peak_2 4344146 4360314 16169 2
peak_3 4492465 4493735 1271 2
ATAC_geneId ATAC_transcriptId ATAC_distanceToTSS ATAC_ENSEMBL
<character> <character> <numeric> <character>
peak_1 497097 ENSMUST00000070533.4 0 ENSMUSG00000051951
peak_2 19888 ENSMUST00000027032.5 27604 ENSMUSG00000025900
peak_3 20671 ENSMUST00000191939.1 1162 ENSMUSG00000025902
ATAC_SYMBOL ATAC_GENENAME ATAC_anno ATAC_logFC ATAC_logCPM
<character> <character> <character> <numeric> <numeric>
peak_1 Xkr4 X-linked Kx blood gr.. Promoter 0.602984 10.45378
peak_2 Rp1 retinitis pigmentosa.. Intron 0.855748 5.18424
peak_3 Sox17 SRY (sex determining.. Promoter -0.620926 7.13469
ATAC_F ATAC_PValue ATAC_qvalue RNA_Row.names RNA_gene_id
<numeric> <numeric> <numeric> <character> <character>
peak_1 41.78446 6.58453e-08 4.81983e-07 ENSMUSG00000051951 497097
peak_2 3.68371 6.13517e-02 9.28785e-02 <NA> <NA>
peak_3 9.14522 4.12368e-03 8.38540e-03 <NA> <NA>
RNA_SYMBOL RNA_GENENAME RNA_ENSEMBL RNA_geneLength
<character> <character> <character> <integer>
peak_1 Xkr4 X-linked Kx blood gr.. ENSMUSG00000051951 457017
peak_2 <NA> <NA> <NA> <NA>
peak_3 <NA> <NA> <NA> <NA>
RNA_geneChr RNA_geneStart RNA_geneEnd RNA_geneStrand RNA_geneId
<character> <integer> <integer> <character> <character>
peak_1 chr1 3214482 3671498 - 497097
peak_2 <NA> <NA> <NA> <NA> <NA>
peak_3 <NA> <NA> <NA> <NA> <NA>
RNA_transcriptId RNA_anno RNA_logFC RNA_logCPM RNA_F
<character> <character> <numeric> <numeric> <numeric>
peak_1 ENSMUST00000070533.4 Gene 1.37498 5.62452 33.7077
peak_2 <NA> <NA> NA NA NA
peak_3 <NA> <NA> NA NA NA
RNA_PValue RNA_qvalue H3K4me3_annotation H3K4me3_geneChr
<numeric> <numeric> <character> <integer>
peak_1 0.00224273 0.00628871 Promoter (<=1kb) 1
peak_2 NA NA <NA> <NA>
peak_3 NA NA <NA> <NA>
H3K4me3_geneStart H3K4me3_geneEnd H3K4me3_geneLength
<integer> <integer> <integer>
peak_1 3214482 3671498 457017
peak_2 <NA> <NA> <NA>
peak_3 <NA> <NA> <NA>
H3K4me3_geneStrand H3K4me3_geneId H3K4me3_transcriptId
<integer> <character> <character>
peak_1 2 497097 ENSMUST00000070533.4
peak_2 <NA> <NA> <NA>
peak_3 <NA> <NA> <NA>
H3K4me3_distanceToTSS H3K4me3_ENSEMBL H3K4me3_SYMBOL
<numeric> <character> <character>
peak_1 0 ENSMUSG00000051951 Xkr4
peak_2 NA <NA> <NA>
peak_3 NA <NA> <NA>
H3K4me3_GENENAME H3K4me3_anno H3K4me3_logFC H3K4me3_logCPM
<character> <character> <numeric> <numeric>
peak_1 X-linked Kx blood gr.. Promoter 0.607114 9.26749
peak_2 <NA> <NA> NA NA
peak_3 <NA> <NA> NA NA
H3K4me3_F H3K4me3_PValue H3K4me3_qvalue H3K4me1_annotation
<numeric> <numeric> <numeric> <character>
peak_1 135.443 4.65079e-31 1.28968e-29 <NA>
peak_2 NA NA NA <NA>
peak_3 NA NA NA <NA>
H3K4me1_geneChr H3K4me1_geneStart H3K4me1_geneEnd H3K4me1_geneLength
<integer> <integer> <integer> <integer>
peak_1 <NA> <NA> <NA> <NA>
peak_2 <NA> <NA> <NA> <NA>
peak_3 <NA> <NA> <NA> <NA>
H3K4me1_geneStrand H3K4me1_geneId H3K4me1_transcriptId
<integer> <character> <character>
peak_1 <NA> <NA> <NA>
peak_2 <NA> <NA> <NA>
peak_3 <NA> <NA> <NA>
H3K4me1_distanceToTSS H3K4me1_ENSEMBL H3K4me1_SYMBOL H3K4me1_GENENAME
<numeric> <character> <character> <character>
peak_1 NA <NA> <NA> <NA>
peak_2 NA <NA> <NA> <NA>
peak_3 NA <NA> <NA> <NA>
H3K4me1_anno H3K4me1_logFC H3K4me1_logCPM H3K4me1_F H3K4me1_PValue
<character> <numeric> <numeric> <numeric> <numeric>
peak_1 <NA> NA NA NA NA
peak_2 <NA> NA NA NA NA
peak_3 <NA> NA NA NA NA
H3K4me1_qvalue H3K27me3_annotation H3K27me3_geneChr H3K27me3_geneStart
<numeric> <character> <integer> <integer>
peak_1 NA <NA> <NA> <NA>
peak_2 NA <NA> <NA> <NA>
peak_3 NA Promoter (<=1kb) 1 4492465
H3K27me3_geneEnd H3K27me3_geneLength H3K27me3_geneStrand
<integer> <integer> <integer>
peak_1 <NA> <NA> <NA>
peak_2 <NA> <NA> <NA>
peak_3 4493735 1271 2
H3K27me3_geneId H3K27me3_transcriptId H3K27me3_distanceToTSS
<character> <character> <numeric>
peak_1 <NA> <NA> NA
peak_2 <NA> <NA> NA
peak_3 20671 ENSMUST00000191939.1 0
H3K27me3_ENSEMBL H3K27me3_SYMBOL H3K27me3_GENENAME
<character> <character> <character>
peak_1 <NA> <NA> <NA>
peak_2 <NA> <NA> <NA>
peak_3 ENSMUSG00000025902 Sox17 SRY (sex determining..
H3K27me3_anno H3K27me3_logFC H3K27me3_logCPM H3K27me3_F
<character> <numeric> <numeric> <numeric>
peak_1 <NA> NA NA NA
peak_2 <NA> NA NA NA
peak_3 Promoter -1.0597 9.04668 87.0802
H3K27me3_PValue H3K27me3_qvalue H3K27ac_annotation H3K27ac_geneChr
<numeric> <numeric> <character> <integer>
peak_1 NA NA Promoter (<=1kb) 1
peak_2 NA NA <NA> <NA>
peak_3 1.32026e-20 5.28858e-18 <NA> <NA>
H3K27ac_geneStart H3K27ac_geneEnd H3K27ac_geneLength
<integer> <integer> <integer>
peak_1 3214482 3671498 457017
peak_2 <NA> <NA> <NA>
peak_3 <NA> <NA> <NA>
H3K27ac_geneStrand H3K27ac_geneId H3K27ac_transcriptId
<integer> <character> <character>
peak_1 2 497097 ENSMUST00000070533.4
peak_2 <NA> <NA> <NA>
peak_3 <NA> <NA> <NA>
H3K27ac_distanceToTSS H3K27ac_ENSEMBL H3K27ac_SYMBOL
<numeric> <character> <character>
peak_1 0 ENSMUSG00000051951 Xkr4
peak_2 NA <NA> <NA>
peak_3 NA <NA> <NA>
H3K27ac_GENENAME H3K27ac_anno H3K27ac_logFC H3K27ac_logCPM
<character> <character> <numeric> <numeric>
peak_1 X-linked Kx blood gr.. Promoter 1.25269 8.81735
peak_2 <NA> <NA> NA NA
peak_3 <NA> <NA> NA NA
H3K27ac_F H3K27ac_PValue H3K27ac_qvalue name
<numeric> <numeric> <numeric> <character>
peak_1 151.496 1.67395e-34 4.0813e-32 peak_1
peak_2 NA NA NA peak_2
peak_3 NA NA NA peak_3
-------
seqinfo: 21 sequences from an unspecified genome; no seqlengthsNormalized metrics for plotting
Recommendations based on ENCODE Project Consortium and other literature:
| Assays | Typical Window Size (± kb) | Reason / Notes |
|---|---|---|
| ATAC-seq | ±1 kb | Narrow peaks, accessibility centered on summit |
| H3K4me3 | ±1 kb to ±2 kb | Promoter mark, sharp and narrow peaks |
| H3K4me1 | ±2 kb to ±5 kb | Enhancer-associated, moderately broad |
| H3K27me3 | ±5 kb to ±10 kb | Broad repressive domains |
| H3K27ac | ±2 kb to ±5 kb | Active enhancers/promoters, broader peaks |
We can check the mean and median of each histone marks and make the normalizedMatrix accordingly.
# Calculate mean and median widths for each dataset
summary_table <- tibble(
Assay = c("ATAC-seq", "H3K4me3", "H3K4me1", "H3K27me3", "H3K27ac"),
Mean_Width = c(
mean(width(rd_atac)),
mean(width(rd_h3k4me3)),
mean(width(rd_h3k4me1)),
mean(width(rd_h3k27me3)),
mean(width(rd_h3k27ac))
),
Median_Width = c(
median(width(rd_atac)),
median(width(rd_h3k4me3)),
median(width(rd_h3k4me1)),
median(width(rd_h3k27me3)),
median(width(rd_h3k27ac))
),
Min_Width = c(
min(width(rd_atac)),
min(width(rd_h3k4me3)),
min(width(rd_h3k4me1)),
min(width(rd_h3k27me3)),
min(width(rd_h3k27ac))
),
Max_Width = c(
max(width(rd_atac)),
max(width(rd_h3k4me3)),
max(width(rd_h3k4me1)),
max(width(rd_h3k27me3)),
max(width(rd_h3k27ac))
)
)
print(summary_table)# A tibble: 5 × 5
Assay Mean_Width Median_Width Min_Width Max_Width
<chr> <dbl> <dbl> <int> <int>
1 ATAC-seq 956. 932 151 3490
2 H3K4me3 1316. 749 191 18723
3 H3K4me1 791. 563 171 12590
4 H3K27me3 1170. 568 176 23571
5 H3K27ac 914. 618 171 22276As we restricted the data for this course to just 2 chromosomes, maximum peaks are around 1kb wide. Hence, we will go ahead with +/- 1kb windows for our analysis.
Taking 1000 bp around the mid of ATAC-peaks
# Extract midpoints of peaks to create windows for visualization
mid_peaks <- gr.mid(overlap)ATAC
# Load ATAC bigWig files
atac_files <- list.files("data", pattern = "ATAC", full.names = TRUE)
names(atac_files) <- gsub(pattern = "\\.bw", replacement = "", x = basename(atac_files))
atac_bw <- lapply(atac_files, function(x){
a <- rtracklayer::import(x)
a <- a[seqnames(a) %in% c("chr1", "chr2")]
a
})
# Normalize signal around mid_peaks
mat_AS <- lapply(atac_bw, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "score",
include_target = TRUE,
mean_mode = "w0",
w = 20,
smooth = T,
background = 0
)
})
saveRDS(mat_AS, file = "output/mat_atac.rds")ChIP
H3K4me3
h3k4me3_files <- list.files("data", pattern = "H3K4me3", full.names = TRUE)
names(h3k4me3_files) <- gsub(pattern = "\\.bw", replacement = "", x = basename(h3k4me3_files))
h3k4me3_bw <- lapply(h3k4me3_files, function(x){
a <- rtracklayer::import(x)
a <- a[seqnames(a) %in% c("chr1", "chr2")]
a
})
mat_h3k4me3 <- lapply(h3k4me3_bw, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "score",
include_target = TRUE,
mean_mode = "w0",
w = 20,
smooth = T,
background = 0
)
})
saveRDS(mat_h3k4me3, file = "output/mat_h3k4me3.rds")H3K4me1
h3k4me1_files <- list.files("data", pattern = "H3K4me1", full.names = TRUE)
names(h3k4me1_files) <- gsub(pattern = "\\.bw", replacement = "", x = basename(h3k4me1_files))
h3k4me1_bw <- lapply(h3k4me1_files, function(x){
a <- rtracklayer::import(x)
a <- a[seqnames(a) %in% c("chr1", "chr2")]
a
})
mat_h3k4me1 <- lapply(h3k4me1_bw, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "score",
include_target = TRUE,
mean_mode = "w0",
w = 20,
smooth = T,
background = 0
)
})
saveRDS(mat_h3k4me1, file = "output/mat_h3k4me1.rds")H27K4me3
h3k27me3_files <- list.files("data", pattern = "H3K27me3", full.names = TRUE)
names(h3k27me3_files) <- gsub(pattern = "\\.bw", replacement = "", x = basename(h3k27me3_files))
h3k27me3_bw <- lapply(h3k27me3_files, function(x){
a <- rtracklayer::import(x)
a <- a[seqnames(a) %in% c("chr1", "chr2")]
a
})
mat_h3k27me3 <- lapply(h3k27me3_bw, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "score",
include_target = TRUE,
mean_mode = "w0",
w = 20,
smooth = T,
background = 0
)
})
saveRDS(mat_h3k27me3, file = "output/mat_h3k27me3.rds")H3K27ac
h3k27ac_files <- list.files("data", pattern = "H3K27ac", full.names = TRUE)
names(h3k27ac_files) <- gsub(pattern = "\\.bw", replacement = "", x = basename(h3k27ac_files))
h3k27ac_bw <- lapply(h3k27ac_files, function(x){
a <- rtracklayer::import(x)
a <- a[seqnames(a) %in% c("chr1", "chr2")]
a
})
mat_h3k27ac <- lapply(h3k27ac_bw, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "score",
include_target = TRUE,
mean_mode = "w0",
w = 20,
smooth = T,
background = 0
)
})
saveRDS(mat_h3k27ac, file = "output/mat_h3k27ac.rds")RNA
# Match overlapping peak names with RNA-seq gene names
tmp <- elementMetadata(overlap)[,c("RNA_Row.names", "name")]
# Get logCPM matrix
counts <- assay(rna, "logCPM")
counts <- assay(rna, "logCPM") - rowMeans(
assay(rna, "logCPM")[, grep(pattern = "11half", x = colnames(assay(rna, "logCPM")),
value = T)]
)
# Merge gene expression values with peaks
mat_RNA <- merge(tmp, counts, by.x = "RNA_Row.names", by.y = "row.names", all.x = T)
# Set rownames to peak names and cleanup
rownames(mat_RNA) <- mat_RNA$name
mat_RNA <- mat_RNA[,-c(1:2)]
colnames(mat_RNA) <- gsub(pattern = ".tsv.gz", replacement = "", x = colnames(mat_RNA))
mat_RNA <- data.matrix(mat_RNA)
mat_RNA <- mat_RNA[names(overlap),]
# Save data
saveRDS(mat_RNA, file = "output/mat_rna.rds")WGBS
# Bisulfite seq coverage files after methylation call
bs_files <- list.files("data", pattern = "WGBS", full.names = TRUE)
names(bs_files) <- gsub(pattern = "\\.bed.gz", replacement = "", x = basename(bs_files))
# Reading coverage
bs_cov <- lapply(bs_files, function(x) {
GRanges(
fread(
input = x,
sep = " ", quote = F, stringsAsFactors = F,
data.table = FALSE, nThread = parallel::detectCores(), showProgress = F,
col.names = c("seqnames", "start", "end", "cov", "Me", "Un", "meth")
)
)
})
# Normalized matrics
mat_bs <- lapply(bs_cov, FUN = function(x) {
normalizeToMatrix(x, mid_peaks,
extend = 1000,
value_column = "meth",
include_target = TRUE,
smooth = TRUE,
mean_mode = "absolute",
background = NA
)
})All signal values are within [0, 1], so we assume it is methylation
signal. Automatically set limit [0, 1] to the smoothed values. If this
is not the case, set argument `limit = NA` in the function to remove
the limits. Set `verbose = FALSE` to turn off this message.saveRDS(mat_bs, file = "output/mat_bs.rds")Question 1
Make an EnrichedHeatmap of methylation data.
EnrichedHeatmap(
mat = mat_bs$WGBS_11half, # normalized matrix
name = "E11.5", # Name for the plot
width = unit(4, "cm"), # Width of the heatmap
height = unit(8, "cm") # Height of the heatmap
) + EnrichedHeatmap(
mat = mat_bs$WGBS_15half, # normalized matrix
name = "E15.5", # Name for the plot
width = unit(4, "cm"), # Width of the heatmap
height = unit(8, "cm") # Height of the heatmap
)The automatically generated colors map from the 1^st and 99^th of the
values in the matrix. There are outliers in the matrix whose patterns
might be hidden by this color mapping. You can manually set the color
to `col` argument.
Use `suppressMessages()` to turn off this message.
The automatically generated colors map from the 1^st and 99^th of the
values in the matrix. There are outliers in the matrix whose patterns
might be hidden by this color mapping. You can manually set the color
to `col` argument.
Use `suppressMessages()` to turn off this message.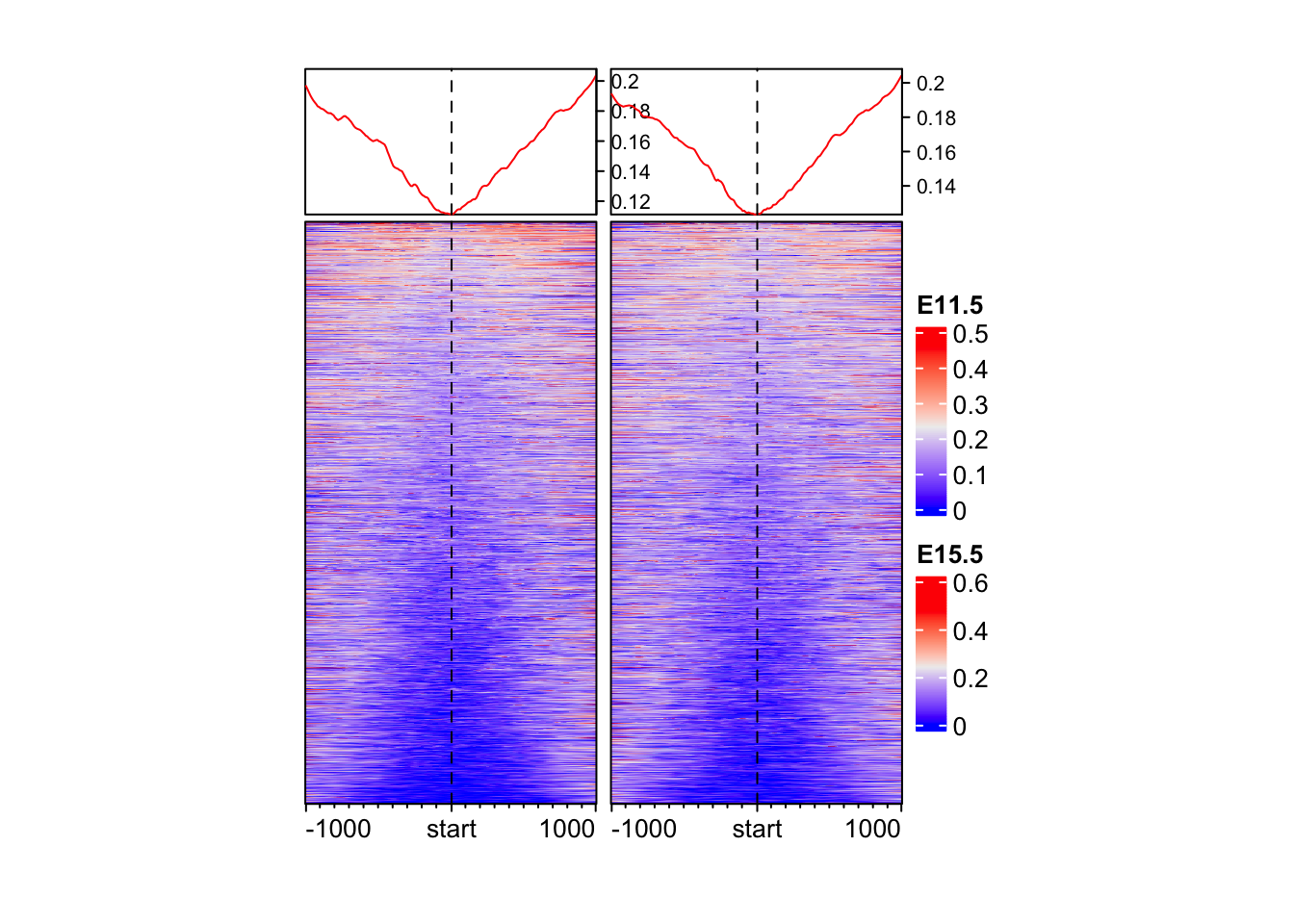
We have not performed differential analysis for DNA methylation data. If you want, you can. Here, we are using DNA methylation as an observartory mark.